So recently I published my first review, where we took a look at how supramolecular hydrogels can benefit the formation of new tissue in the body. I’ll summarize the paper here, and tell a bit about how it came to be. For the original source in Acta Biomaterialia, take a look here: https://doi.org/10.1016/j.actbio.2021.01.034.
.
I’ve always been fascinated with tissue integration and how cells remodel the extracellular matrix during regeneration. How do they do it? What do they need to do it? Are they anything like myself when I remodel my flat?

Hypothetically, if a cell wants to remodel their living space, then I would guess they need some freedom to move (open space), the ability to reorganize their surroundings (not too heavy furniture), and the desire (trigger) to do so.
This thought process led us to try and understand one of the most important design components of new tissue engineering implants: tissue integration. Supramolecular materials, in particular, act as these remodelable environments due to their non-covalent interactions.

Tissue Integration
In order to fulfill the promises of tissue engineering and regenerative medicine, we need to develop materials that allow cells to not only form new functional tissue in the body but also integrate with the existing tissue. We like to think of this as tissue integration. The ability of a matrix or material to both provide cells the right cues to form tissue and the ability to allow the interface between the newly formed and existing tissue to merge.
We are firm believers, not to be confused with beliebers, that cells need a scaffold, material, or 3D shape in order to guide their behavior and form functional tissue. Naturally, the materials outside of the cell (the extracellular matrix or ECM) play an enormous role in the communication between cells and provides the proper cues to cells and tissues instructing them what to do. To be honest, as Chemists, we like to try to recreate properties of this natural ECM – this gives us something to play around with.
Engineering a material’s degradation profile has largely been the integrative route of choice. For example, degradation by hydrolysis and MMP cleavable sites have been introduced into hydrogel formulations to enhance cellular takeover and ECM production.

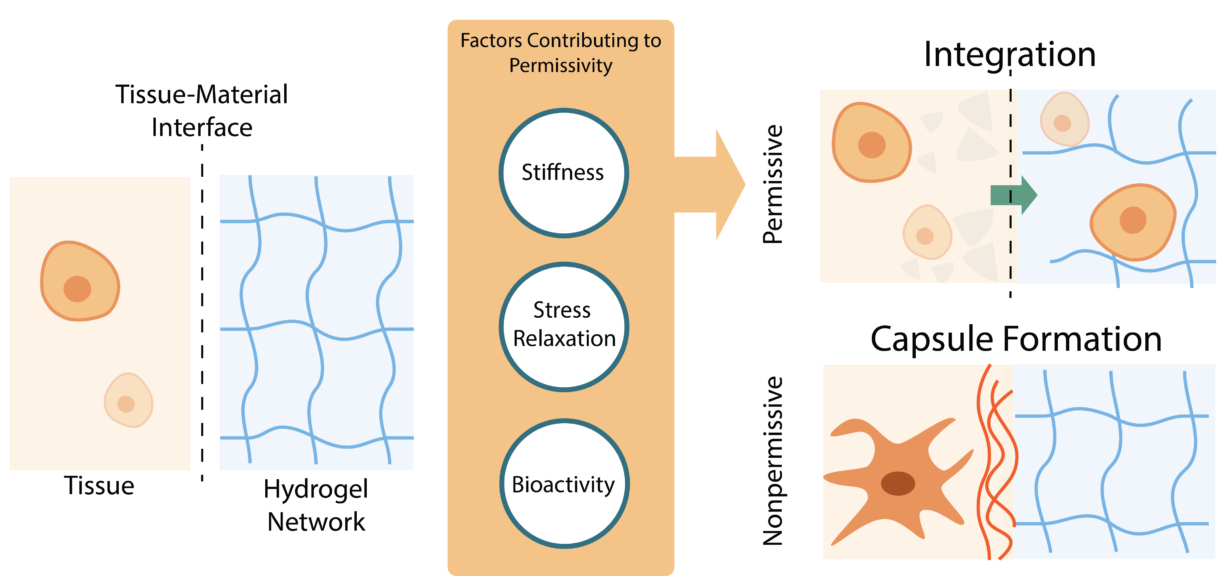
In MMP degradable hydrogels, the niche is broken down so that a new matrix can be built by the cells. Due to the reversibility of supramolecular moieties, a new route to integration and remodelability is possible that doesn’t require degradation of the material. Moieties that utilize hydrogen bonds, host-guest interactions and pi-pi stacking as some examples self-assemble into supramolecular hydrogels. Three factors, stiffness, stress relaxation, and bioactivity, contribute to how permissive these supramolecular hydrogel materials are to cells (Figure 1). When a construct is not engineered as permissive, cellular takeover is inhibited and thus may result in an immune reaction or foreign body response. One can imagine an open and airy flat with a modular conceptual layout.

In our review, we paid special attention to supramolecular systems with proven tissue integrative properties. Thus, we classified three main types (hydrogen bonding, host-guest, and peptide amphiphiles) that not only have shown reversibility and supramolecular behavior in-situ but also after in-vivo implantation. We will highlight these below to present examples of their proven tissue integration. Also in the review, we expanded on how to characterize and design a supramolecular system according to its mechanical properties (stiffness, relaxation) and bioactive properties. For more detail on how to characterize and how to engineer these systems, we direct you to our review itself, which compares static cues to dynamic cues along with added benefits of supramolecular hydrogels including, biomimicry, self-healing, injectability, and responsiveness.

UPys
Already well-studied in cartilage and myocardial applications, 2-ureido-4[1H]-pyrimidinone (UPy) is a popular motif that was made popular by Meijer and Dankers. In Figure 2, picrosirious red staining shows that with PEGdiUPy hydrogel (infused with VEGF and IGF1) there is reduced fibrosis after injury. This indicates better tissue regeneration than without the hydrogel. In fact, in previous work, they found myocardial cells within the hydrogel matrix. Biomaterials comprised of supramolecular UPys have shown responsiveness, injectability, and modularity that make them highly versatile in bioengineering.
.
Host/Guest
Under the host/guest umbrella are two different macrocycles (cyclodextrin and cucubtrils) that can be used to complex polymer chains and render a physical hydrogel. Webber’s lab synthesized hydrogels made from cucubtrils that spanned a range of affinities resulting in tailorable physical properties. Hydrogels made from Keq =1.5×107 M-1 were found to be more permissive than tougher, higher Keq gels, with more cells infiltrated and ECM produced after implantation. These showcased a tunable level of integration that could be useful in a variety of tissue applications.
.
Peptide Amphiphiles
Hydrogels can also be made using peptide amphiphiles (PA) which have been researched in considerable detail by the Stupp lab. In nerve applications, the PA hydrogel was synthesized as material to fill in long gap injuries. Schwann cells were found to spread on the PA gel and after implantation, these cells were found to infiltrate the scaffold. PAs have also been used in a number of tissues from neural, skin, to cartilage and found to be especially useful in soft tissue applications.
.
Others
While these aforementioned classes have shown excellent tissue integration, other classes are being developed for a similar purpose. For example hydrogels made from benzene-1,3,5-tricarboxamide, collagen mimetic peptides, etc will hopefully soon show similar performance as implantable materials. Supramolecular hydrogels can be rationally designed according to stiffness, relaxation, and bioactive properties. In addition, these materials can be injected or 3D printed. These “complex” systems can also be engineered to be permissive whereby cells move inside the material and begin the process of regeneration. They represent an emerging class of materials that are fundamentally different from classically degradable hydrogels. We hope with our review you too are inspired by supramolecular interactions and find them useful with many benefits for non-invasive tissue engineering applications.
.
Our Process
This project started out as an invitation to write a piece on something we found interesting with supramolecular biomaterials. Remodeling my own flat was particularly important during the lockdown, can you tell? Our lab is inspired by dynamic biomaterials and the fundamental cell interactions behind remodeling and integration. At the beginning of lockdown, Matt gave me freedom to explore these ideas and I pitched integration via supramolecular hydrogels. While it took some time to focus this idea, we eventually found a nice story to tell. Older literature shows the importance of degradation, but supramolecular and dynamic strategies have been largely overlooked for their permissivity that allows integration to occur within the material itself. With the idea in hand, we made some figures, sketched out ideas and started writing. We didn’t want this to be a comprehensive review, but moreso to showcase a few of the more “famous” motifs and how they have shown this integration in-vivo. I will say that this was a bit of an issue in the review phase, where reviewers asked for a more comprehensive viewpoint. Luckily, we were able to convince them of the power of simplicity—adding a plethora of examples would not have strengthened our message. We paid attention to those examples where clear integration was shown. Overall, we took a different approach to write this review, and we learned a lot along the way (cells are very particular about their living room setup). We really hope this gets other labs documenting the tissue integration, and spurs labs to figure out ways to predict this in-vitro as a standard development test.